Monodelphis domestica, the gray short-tailed opossum, is native to Brazil, Bolivia, Paraguay, and Argentina, but has found its way to North America as a common biomedical laboratory test animal. Monodelphis is a member of Didelphidae, a group of exclusively New World opossums thought to be basal among the living marsupials.
Didelphids are often used to approximate basal mammals because they retain several osteological features considered primitive for Mammalia, and are characterized by relatively few derived characters. Within Didelphidae, Monodelphis is most closely related a clade of small-bodied taxa including Marmosa, the mouse opossum. A more familiar and more distant cousin of M. domestica is the Virginia opossum, Didelphis virginiana. These two taxa are distinguishable in a number of ways: Monodelphis is approximately 50 times smaller in body size than Didelphis, and lacks a pouch which is found in the latter. Furthermore, the skulls of the two opossums vary in several ways (see Macrini, 2000).
Development in marsupials is of particular interest because many of the features that originate embryonically in placental mammals do not appear until after birth in marsupials. Thus, development of systems such as the skeleton are easier to study in marsupials making growth series such as this one invaluable to the study of mammalian evolution, and the relationship between ontogeny and phylogeny (Clark and Smith, 1993; Macrini, 2000).
Monodelphis is an excellent taxon to study postnatal development of the mammalian skeleton because the young are born 14 days after conception. At birth (day 0 in the growth series), very little of the skeleton is ossified -- only portions of the dentary. The growth series scanned and displayed here ranges from day 27 postnatal to an adult (usually older than day 120, based on its fully erupted dentition).
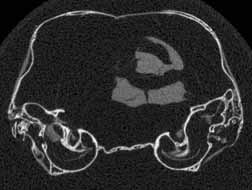
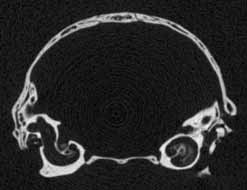
Several features of mammalian development are visible in members of the growth series shown on this website. An obvious change is the thickening of the skull elements as ontogeny progresses -- particularly the bones that surround the braincase and the ear region (contrast the thickness of the bones in the unreduced coronal slice #401 in day 27 and the unreduced coronal slice #390 in day 90, shown below). A feature associated with the thickening of the skull bones is the closure of the parietal fontenelle between days 48 and 57.
 |
 |
|
Reduced coronal slice #401 through the braincase of day 27 specimen. Click on the thumbnail for an unreduced version. |
Reduced coronal slice #390 through the braincase of day 90 specimen. Click on the thumbnail for an unreduced version. |
Another notable feature is the reduction in size of the petrosals relative to the rest of the braincase through ontogeny. This is due to changes in the size and shape of the braincase relative to the petrosal size, which remains fairly constant in size throughout postnatal ontogeny. Also, the eruption of the dentition can easily be studied from the CT scans, including the marsupial replacement pattern of the deciduous third premolar only. Events not well described in mammalian development, such as the relationship of the mental foramen to the roots of the erupting and replacing dentition, can be examined in detail using the CT imagery (see Macrini, 2000 for a description of this relationship in the adult Monodelphis).

Literature
Archer, M. 1976. The basicranial region of marsupicarnivores (Marsupialia), interrelationships of carnivorous marsupials, and the affinities of the insectivorous marsupial peramelids. Zoological Journal of the Linnean Society 59:217-322.
Broom, R. 1909. Observations on the development of the marsupial skull. Proceeding of the Linnean Society of New South Wales 34:195-214.
Clark, C. T., and K. K. Smith. 1993. Cranial osteogenesis in Monodelphis domestica (Didelphidae) and Macropus eugenii (Macropodidae). Journal of Morphology 215:119-149.
de Beer, G. 1937. The development of the vertebrate skull. Clarendon Press, Oxford, 552pp.
Evans, H. E. 1993. Miller's anatomy of the dog. 3rd edition. Philadelphia: W. B. Saunders Company, 1113 pp.
Filan, S. 1991. Development of the middle ear region in Monodelphis domestica (Marsupialia, Didelphidae): marsupial solutions to an early birth. Journal of Zoology (London) 225:577-588.
Gray, H. 1977. Anatomy, descriptive and surgical. 15th edition. New York: Crown Publishers, INC., 1257 pp.
Kemp, T. S. 1982. Mammal-like reptiles and the origin of mammals. Academic Press, New York, 363 pp.
Larsell, O., E. McCrady, Jr., and A. A. Zimmermann. 1935. Morphological and functional development of the membraneous labyrinth in the opossum. Journal of Comparative Neurology 63:95-118.
MacIntyre, G. T. 1972. The trisulcate petrosal pattern of mammals. In: T. Dobzhansky, M. K. Hecht, and W. C. Steere (eds.), Evolutionary biology. Vol. 6. Appleton-Century-Crofts, New York, pp. 275-303.
Macrini, T. E. 2000. High resolution X-ray computed tomography (CT) of the skull of an extant opossum (Monodelphis domestica) and a comparison of its ontogeny to synapsid phylogeny. Unpublished M.S. thesis, University of Texas, Austin, Texas, 158 pp.
Macrini, T. E. 2004. Monodelphis domestica. Mammalian Species 760:1-8.
Maier, W. 1987. The ontogenetic development of the orbitotemporal region of the skull of Monodelphis domestica (Didelphidae, Marsupialia), and the problem of the mammalian alisphenoid. In: H. J. Kuhn and U. Zeller (eds.), Mammalia depicta, morphogenesis of the mammalian skull. Paul Parey Verlag, Hamburg, pp. 71-90.
________. 1987. Der processus angularis bei Monodelphis domestica (Didelphidae; Marsupialia) und seine beziehungen zum mittelohr: eine ontogenetische und evolutionmorphologische untersuchung. Gegenbaurs morphologisches Jahrbuch 133:123-161.
Meng, J., and R. C. Fox. 1995. Osseous inner ear structures and hearing in early marsupials and placentals. Zoological Journal of the Linnean Society 115:47-71.
McClain, J. A. 1939. The development of the auditory ossicles of the opossum (Didelphis virginiana). Journal of Morphology 64:211-265.
Murray, P., R. Wells, and M. Plane. 1987. The cranium of the Miocene thylacoleonid, Wakaleo vanderleuri: go to the shears- a fresh bite at thylacoleonid systematics. In: M. Archer (ed.), Possums and opossums: studies in evolution. Vol. 2. Surrey Beatty and Sons Pty Limited, Sydney, pp. 433-466.
Negus, V. 1958. The comparative anatomy and physiology of the nose and paranasal sinuses. E. S. Livingstone Ltd., London, 402 pp.
Novacek, M. J. 1993. Patterns of diversity in the mammalian skull. In: J. Hanken and B. K. Hull (eds.), The skull: patterns of structural and systematic diversity. Vol. 2. University of Chicago Press, Chicago, pp.438-545.
Reig, O. A., J. A. W. Kirsch, and L. G. Marshall. 1987. Systematic relationships of the living and Neocenozoic American "opossum-like" marsupials (Suborder Didelphimorphia), with comment on the classification of these and of the Cretaceous and Paleogene new world and European Metatherians. In: M. Archer (ed.), Possums and opossums: studies in evolution. Vol. 1. Surrey Beatty and Sons Pty Limited, Sydney, pp. 1-89.
Rowe, T. 1996. Brain heterochrony and the origin of the mammalian middle ear. Memoirs of the California Academy of Sciences 20:71-95.
_______, W. Carlson, and W. Bottorff. 1993. Thrinaxodon: digital atlas of the skull. University of Texas Press, Austin (2nd edition, CD-ROM for MS Windows/ Macintosh platforms).
Smith, K. K. 1994. Development of the craniofacial musculature in Monodelphis domestica (Marsupialia, Didelphidae). Journal of Morphology 222:149-173.
Weil, R. 1899. Development of the ossicula audita in the opossum. Annals of the New York Academy of Sciences 12:103-113.
Wible, J. R. 1990. Petrosals of late Cretaceous marsupials from North America, and a cladistic analysis of the petrosal in therian mammals. Journal of Vertebrate Paleontology 10:183-205.
_________, and J. A. Hopson. 1993. Basicranial evidence for early mammal phylogeny. In: F. S. Szalay, M. J. Novacek, M. C. McKenna (eds.), Mammal phylogeny: Mesozoic differentiation, multituberculates, monotremes, early therians, and marsupials. Vol. 1. Springer-Verlag, New York, pp. 45-62.
Links
Southwest Foundation for Biomedical Research
The National Opossum Society
Didelphis virginiana on The Animal Diversity Web (The University of Michigan Museum of Zoology)
The brain of Didelphis virginiana (Comparative Mammalian Brain Collections website)
Didelphis virginiana on The Mammals of Texas Online Edition
Sakaguchi Lab (Iowa State University)
Smith Lab (Duke University)


